Frontiers in Human Neuroscience 01 frontiersin.org
The impact of physical therapy on
dysphagia in neurological
diseases: a review
KunLi
1
*, CuiyuanFu
1
, ZhenXie
1
, JiajiaZhang
2
,
ChenchenZhang
1
, RuiLi
1
, CaifengGao
1
, JiahuiWang
1
,
ChuangXue
3
, YuebingZhang
1
and WeiDeng
3,4
*
1
Shandong Daizhuang Hospital, Jining, China,
2
Department of Psychology, Xinxiang Medical
University, Xinxiang, China,
3
Aliated Mental Health Center and Hangzhou Seventh People’s Hospital,
Zhejiang University School of Medicine, Hangzhou, China,
4
Liangzhu Laboratory, MOE Frontier
Science Center for Brain Science and Brain-machine Integration, State Key Laboratory of Brain-
Machine Intelligence, Zhejiang University, Hangzhou, China
A neurogenic dysphagia is dysphagia caused by problems with the central
and peripheral nervous systems, is particularly prevalent in conditions such
as Parkinson’s disease and stroke. It significantly impacts the quality of life
for aected individuals and causes additional burdens, such as malnutrition,
aspiration pneumonia, asphyxia, or even death from choking due to improper
eating. Physical therapy oers a non-invasive treatment with high ecacy
and low cost. Evidence supporting the use of physical therapy in dysphagia
treatment is increasing, including techniques such as neuromuscular electrical
stimulation, sensory stimulation, transcranial direct current stimulation, and
repetitive transcranial magnetic stimulation. While initial studies have shown
promising results, the eectiveness of specific treatment regimens still requires
further validation. At present, there is a lack of scientific evidence to guide
patient selection, develop appropriate treatment regimens, and accurately
evaluate treatment outcomes. Therefore, the primary objectives of this review
are to review the results of existing research, summarize the application of
physical therapy in dysphagia management, wealso discussed the mechanisms
and treatments of physical therapy for neurogenic dysphagia.
KEYWORDS
Parkinson’s disease, stroke, schizophrenia, dysphagia, neuromuscular electrical
stimulation, repetitive transcranial magnetic stimulation, sensory stimulation,
transcranial direct current stimulation
1 Introduction
ere are several complex physiological movements involved in swallowing, including
movements of the mouth, pharynx, larynx, and esophagus (Shaw and Martino, 2013).
Dysphagia refers to the disruption of the normal swallowing process (Rofes etal., 2011), which
poses severe risks including malnutrition, aspiration pneumonia, asphyxia, etc (Hurtte etal.,
2023). e causes of dysphagia can be divided into neurogenic, structural, and mental
dysphagia (Medicine DRCO, 2023). A neurogenic dysphagia results from problems with the
central and peripheral nervous systems (El Halabi etal., 2023). e number of people suering
from neurogenic dysphagia each year worldwide is estimated at 400000 to 800,000 (Panebianco
et al., 2020). Among the diseases that predispose to neurogenic dysphagia are stroke,
OPEN ACCESS
EDITED BY
Elisa Kallioniemi,
New Jersey Institute of Technology,
UnitedStates
REVIEWED BY
Maja Rogić Vidaković,
University of Split, Croatia
Anna-Lisa Schuler,
Max Planck Institute for Human Cognitive and
Brain Sciences, Germany
*CORRESPONDENCE
Kun Li
Wei Deng
RECEIVED 28 March 2024
ACCEPTED 28 May 2024
PUBLISHED 06 June 2024
CITATION
Li K, Fu C, Xie Z, Zhang J, Zhang C,
Li R, Gao C, Wang J, Xue C, Zhang Y and
Deng W (2024) The impact of physical
therapy on dysphagia in neurological
diseases: a review.
Front. Hum. Neurosci. 18:1404398.
doi: 10.3389/fnhum.2024.1404398
COPYRIGHT
© 2024 Li, Fu, Xie, Zhang, Zhang, Li, Gao,
Wang, Xue, Zhang and Deng. This is an
open-access article distributed under the
terms of the Creative Commons Attribution
License (CC BY). The use, distribution or
reproduction in other forums is permitted,
provided the original author(s) and the
copyright owner(s) are credited and that the
original publication in this journal is cited, in
accordance with accepted academic
practice. No use, distribution or reproduction
is permitted which does not comply with
these terms.
TYPE Review
PUBLISHED 06 June 2024
DOI 10.3389/fnhum.2024.1404398
Li et al. 10.3389/fnhum.2024.1404398
Frontiers in Human Neuroscience 02 frontiersin.org
Parkinson’s disease, amyotrophic lateral sclerosis, multiple sclerosis,
and other forms of neurodegeneration (Chandran and Doucet, 2024).
It is a common complication of stroke to experience dysphagia
aerward, it is estimated that 20–43% of patients have persistent
dysphagia aer 3 months, which can lead to aspiration pneumonia,
malnutrition, water and electrolyte disorders, and other complications
(Chen etal., 2024). ere is a prevalence of 18–100% of Parkinson’s
disease with dysphagia, it can lead to dehydration, malnutrition,
aspiration pneumonia, depression, and social isolation, and it can also
aect the quality of life and even cause death (Dashtelei etal., 2024).
Dehydration, malnutrition, asphyxia, and death are all risks associated
with neurogenic dysphagia, which severely reduces the quality of life
for the patient (Xia etal., 2023; Kocica etal., 2024).
Physical therapy, as a new treatment method, directly targets the
swallowing nerve circuit to enhance swallowing function (Li etal.,
2023). Common clinical treatments for dysphagia include
neuromuscular electrical stimulation (NMES), sensory stimulation,
repetitive transcranial magnetic stimulation (rTMS), and transcranial
direct current stimulation (tDCS) (Alvarez-Berdugo et al., 2016;
Miller etal., 2022; Li etal., 2023). However, there remains paucity of
discourse on the application of physical therapy in dysphagia
management in dierent diseases. e purpose of this review article
is to provide more theoretical support for the application of physical
therapy in neurogenic dysphagia, and to describe treatment principles
and treatment programs of several commonly used physical
therapy programs.
2 Mechanisms associated with
dysphagia
Swallowing is a complex process, it involves the coordination of
more than 30 muscles in the mouth, pharynx, larynx, and esophagus,
encompassing four distinct stages: oral preparation, oral transit,
pharynx, and esophageal phase (Dodds etal., 1990). It involves various
levels of the central nervous system, from the cortex to the medulla, as
well as multiple cranial and peripheral nerves (El Halabi etal., 2023).
It is well recognized that pharyngeal movements are strongly related to
the innervation of sensory branches of the cranial nerves (Nascimento
et al., 2021). Usually, swallowing is controlled by four types of
components: (1) aerent motor bers in cranial nerves and ansa
cervicalis; (2) aerent sensory bers in cranial nerves; (3) bers lining
the cerebral, cerebellar, and cerebellar hemispheres that synapse in the
swallowing centers; (4) paired swallowing centers in the brainstem that
synapse with each other (Dodds et al., 1990). In the swallowing
process, ber transmitters transmit signals from peripheral nerves and
the cerebral cortex to the swallowing centers in the brain stem
(Hashimoto etal., 2019). ere is a complex unit called the swallowing
central pattern generator that is composed of motor neurons and
interneurons located in the brainstem’s medulla oblongata, a region
that contains swallowing neurons (Sasegbon etal., 2024). Two parts
make up the pattern generator of the swallowing center: (1) the dorsal
region consisting of the nucleus tractus solitarius and peripheral
neurons; (2) the nucleus and reticular formation surrounding the
nucleus are located in the ventral region (Jang and Kim, 2021). Nucleus
ambiguous innervates the muscles of the oral cavity, larynx, and
pharynx through the trigeminal, facial, glossopharyngeal, vagus, and
accessory nerves (Petko and Tadi, 2023; Chandran and Doucet, 2024),
e nucleus tractus solitary can receive incoming information from
the nucleus doubtful and then send eerent bers to the corresponding
muscles, but mainly integrates information from higher cortical
centers and peripheral sensory aerents and regulates swallowing
according to the nature of the food bolus (Chandran and Doucet, 2024;
Ye etal., 2024). Overall (see Figure1), the swallowing central pattern
generator is responsible for the formation and regulation of swallowing
motor sequences, processing incoming information, generating
preprogrammed swallowing responses, and distributing appropriate
signals to the motor nuclei of cranial nerves and their axons, which are
ultimately transmitted to the many muscles involved in swallowing
(Yamamoto etal., 2022).
A trigeminal and facial nerve innervate the muscles of the mouth,
masticatory muscles are innervated by the trigeminal nerve, while the
glossopharyngeal nerve and the vagus nerve innervate the muscles of
the pharynx (Costa, 2018). In addition, the contraction of the esophageal
sphincter aects the operation of swallowing, it contains the
cricopharyngeal and hypopharyngeal constrictors and is innervated by
the vagus nerve, while the muscularis that promote the contraction of
the esophageal sphincter during swallowing, such as the suprahyoid
muscles (stylohyoid, digastric, and mylohyoid muscles) and thyrohyoid
muscles, are innervated by the trigeminal, facial, and hypoglossal nerves
(McCarty and Chao, 2021). e recruitment of muscles necessary for
the swallowing sequence is directed by the swallowing network, and
integration between descending signals and aerent inputs may occur
in the cortex and cerebellum, where there are multiple synaptic
connections for dierent functions, the cerebral cortex may
beresponsible for the initiation of motor commands, and some cortical
areas may beresponsible for the integration of chewing and swallowing
information, other regions, however, feed the descending signal back to
the brain stem with the sensation of the bolus moving along the
swallowing channel (Cheng etal., 2022). Although movement is directed
by the cortex, the cerebellum is also associated with movement and plays
a key role in the balanced coordination of muscle movements (Roostaei
etal., 2014). Inuences the cortical swallowing module consisting of
primary motor, auxiliary motor, primary sensory cortical areas, and
cingulate gyrus (Sasegbon and Hamdy, 2023).
e term “neurogenic dysphagia” refers to dysphagia, or
dysfunction of swallowing mechanisms, in patients who have suered
a neurologic insult or disease (Teismann etal., 2007). Such diseases
include stroke, Parkinson’s disease, and multiple sclerosis, among
other neurodegenerative disease processes (Chandran and Doucet,
2024). Dysphagia following stroke primarily stems from cerebral
cortex and subcortical structures damage, aecting areas like the
motor cortex, cerebellum, thalamus, and other parts, as well as sensory
defects of the pharyngeal mucosa (Teismann etal., 2007; Qin etal.,
2023). It is characterized by a delayed or absent swallowing reex and
a premature overow of bolus (Labeit etal., 2023), pharyngeal food
residues and pharyngeal motility disorders (Warnecke etal., 2021). A
dysphagia caused by Parkinson’s disease is dierent from dysphagia
caused by stroke because it is primarily caused by problems with the
brainstem, muscle atrophy, and dopaminergic and non-dopaminergic
mechanisms (Patel et al., 2020). e patient presented with
hypoesthesia of the pharynx, food residue, and bradykinesia of the
oropharynx (Labeit etal., 2020a), the swallowing reex was impaired,
and the bolus overowed prematurely (Labeit et al., 2020b). e
pathological mechanisms of dysphagia in multiple sclerosis include
damage of cortical bulbar bers alone or in combination, damage of

Li et al. 10.3389/fnhum.2024.1404398
Frontiers in Human Neuroscience 03 frontiersin.org
the brainstem swallowing center, abnormalities of the cerebellum
aecting the accuracy of sequential planning and coordination of
swallowing, failure of the aerent nerve central sensory pathway and
abnormal impairment of the central motor pathway (Alfonsi etal.,
2013). Its dysfunction may occur at any stage of swallowing and cause
various complications such as aspiration pneumonia, malnutrition
and airway obstruction (Ansari etal., 2020).
3 Physical therapy
3.1 Neuromuscular electrical stimulation
e purpose of NMES is to stimulate peripheral nerves associated
with paralyzed pharyngeal muscles with low-frequency electrical
stimulation, aiming to enhance their functionality (Doucet et al.,
2012). In simpler terms, the eects of NMES on swallowing are
improved through the contraction of pharyngeal muscles (Carnaby
et al., 2020). Laryngeal elevation and reduction resulting from
pharyngeal muscle defects are the primary causes of dysphagia in
stroke patients, leading to potential issues such as aspiration and
pharyngeal residue (Bath etal., 2018). erefore, the NMES therapy
is considered one of the most eective treatments for dysphagia
caused by stroke (Beom et al., 2011). Moreover, patients with
Parkinson’s disease oen use NMES as a form of physical therapy to
improve tongue muscle weakness (Park etal., 2018).
Preliminary studies indicated that increased tongue power results
in greater activation of the suprahyoid muscle during swallowing (Oh,
2016). is goal can beachieved by NMES, by depolarizing motor axons
and activation of type II fast-twitch muscle bers in neuromuscular
tissues, either through peripheral nerves or muscle belly (Carnaby etal.,
2020; Carson and Buick, 2021). In patients with brain injury-related
dysphagia, the NMES strengthens both suprahyoid and infrahyoid
muscles, along with the muscles that assist in swallowing (Seo etal.,
2021). Moreover, the long-term application of NMES benets the
recovery of swallowing-related cortical neuroplasticity in stroke patients
(Zhang etal., 2022). Given the loss of swallowing motor control in stroke
patients, functional muscle contraction patterns are primarily
re-educated during NMES (Miller etal., 2022). is entails triggering
the peripheral neuromuscular system via external electrical stimulus,
depolarizing cervical muscle nerve bers, and initiating oropharyngeal
muscle contraction to improve swallowing function (Wang etal., 2023).
In addition, it shows promise in improving dysphagia associated with
other diseases such as Parkinson’s disease and head and neck cancer, in
similar ways to how NMES improves dysphagia associated with strokes,
it stimulates the nerve and motor endplates of the nerve (Tan etal., 2013).
In the application of NMES, electrodes are typically positioned on
the hyoid muscles or adjacent areas, utilizing a frequency range of 25
to 120 Hz (Miller etal., 2022). Recent research ndings, as summarized
in Table S1, suggest the optimal treatment duration for NMES is
generally between 20 to 30 min, with an application frequency of
80 Hz, and electrodes placed on the hyoid muscles (Miller etal., 2022).
At present, studies consistently demonstrate that NMES improves
swallowing function in patients suering from neurogenic dysphagia,
attributed to the following factors: (1) stimulation of the tongue,
orbicular oris muscles, and other related muscles to promote the
development of normal movement patterns and enhance organ and
muscle functions; (2) alteration in the excitability of the pharyngeal
cortex to promote normal swallowing mode operation; (3) activation
of the swallowing center, facilitating the functional reconstruction of
the nervous system (Konecny and Elfmark, 2018; Meng etal., 2018;
Zeng etal., 2018; Oh etal., 2020).
FIGURE1
The mechanism of swallowing. As part of the swallowing central program generator, the nucleus ambiguous receives relevant cranial nerve stimulation
and the nucleus tractus solitarius receives cortical and peripheral sensory input, after receiving the signal, the swallowing-related muscles are
stimulated to promote the normal swallowing process (Yamamoto etal., 2022). (A) The swallowing center’s operation. (B) Swallowing muscles. (C) A
normal swallowing process; NTS, Nucleus tractus solitarius; CPG, Central program generator; NA, Nucleus ambiguous; V, Trigeminal nerve; VII, Facial
nerve; IX, Glossopharyngeal nerve; X, Vagus nerve; XI, Accessory nerve.
Li et al. 10.3389/fnhum.2024.1404398
Frontiers in Human Neuroscience 04 frontiersin.org
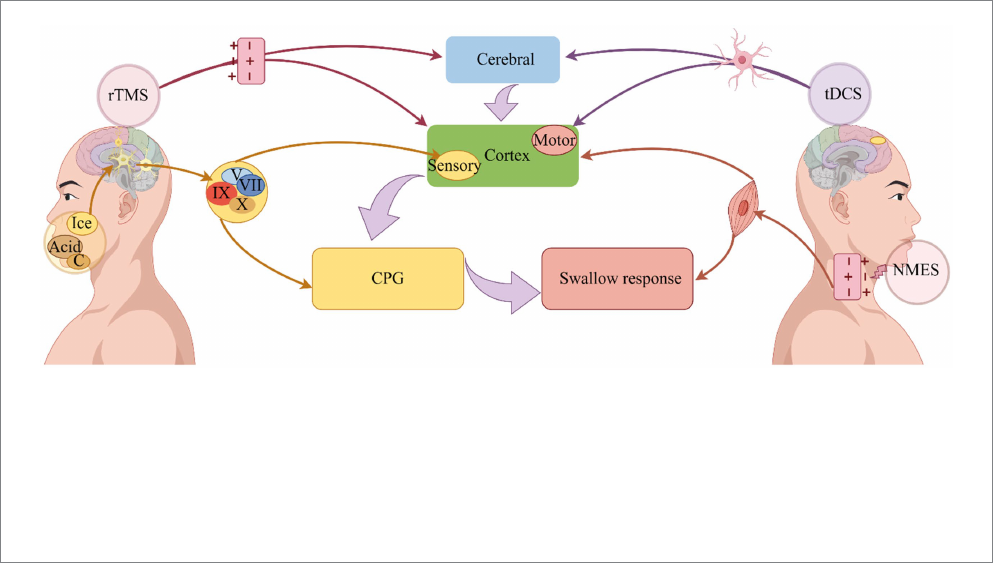
In summary (see Figure2), the NMES stimulates the depolarization
of the axons below the electrode, and the depolarization signal of motor
axons propagates from the stimulation site to the muscle (peripheral
pathway) to produce contraction, which can induce the neural plasticity
of the central nervous system and enhance the neuromuscular function
aer nervous system injury (Bergquist etal., 2011). A NMES consists of
muscle reeducation primarily focused on improving swallowing function
by facilitating normal swallowing mode operation (Jeon etal., 2020).
3.2 Sensory stimulation
Sensory stimulation plays a crucial role in promoting the
rehabilitation of swallowing function (Cola et al., 2012). Normal
swallowing relies on somatosensory inputs associated with trigeminal,
glossopharyngeal, and vagus nerves (Jean, 2001). When the sensory
information pathway is impaired, reduced sensory input can slow
down the swallowing-cortical pathway, resulting in dysphagia
(Teismann etal., 2007). Sensory stimulation of the cranial nerves
enhances the transmission of information to the solitary tract nucleus
in the brainstem (Alvarez-Berdugo etal., 2016). is, in turn, increases
the sensory input to the nucleus tract solitary in the brainstem through
cranial nerves, promoting the operation of normal swallowing pattern,
and ultimately improving swallowing function (Jean, 2001). Common
sensory stimulation methods include ice, acid, and carbonation
stimulation (Regan, 2020).
Ice stimulation therapy uses repetitive mechanical, pressure, and
temperature stimulation to enhance the sensitivity of the so palate
and pharynx. By increasing the sensory sensitivity of local nerves, ice
stimulation prompts local muscles contraction and triggers the
swallowing reex (Li et al., 2017). Consequently, ice stimulation
mobilizes resting neuron excitability, reconstructs the neural network
to achieve functional reorganization, promotes the normal swallowing
reex, and restores the function of swallowing organs (Ilott etal.,
2016). In the application of ice stimulation therapy Nakamura and
Fujishima (2013) dipped a cotton stick about 10 cm long and 1.27 cm
in diameter into the water until it froze into a frozen sucker, then
lightly rubbed and pressed it against the posterior tongue, bottom of
the tongue, and posterior pharyngeal wall of stroke patients with
dysphagia for 10 s, results revealed that ice stick massage could shorten
the threshold of the swallowing response phase. Kawakami et al.
(2019) demonstrated that placing an ice stick in the mouth is superior
to placing it on the neck. Intraoral ice stimulation signicantly can
increase the excitability of the swallowing pathway in the cortex,
trigger swallowing initiation, and shorten the duration of the
pharyngeal phase. Early rehabilitation of stroke patients with dysphagia
is closely related to the central nervous system’s ability to compensate
and reconstruct injured areas, thereby increasing the excitability of the
nervous system and facilitating swallowing by activating the central
nervous system to form new sensory and motor projections (Qin etal.,
2019). At this point, when applied to stroke-related dysphagia, ice
stimulation’s eects primarily manifest in two ways. Firstly, it activates
sensory nerve bers, boosts sensory recovery, and restores the neural
network (Ferrara etal., 2018). Secondly, it enhances sensory sensitivity.
By increasing the sensitivity of the swallowing reex area, it amplies
sensory inputs before swallowing, induces the generation of swallowing
reex, and nally improves swallowing function (Cui etal., 2020).
e improvement of dysphagia through acid stimulation may
beattributed to sensory feedback information (Logemann etal., 1995).
Previous studies investigating neurogenic swallowing dysfunction
found that subjects consuming water with a citric acid concentration of
2.7%, compared to plain water, exhibited increased spontaneous
swallowing and reduced aspirations, leading to an improvement in
swallowing function (Pelletier and Lawless, 2003). Wang etal. (2022)
reported an eective acid stimulation medium for treating dysphagia
in stroke patients. is method entailed applying vitamin C tablet
FIGURE2
Improvement of dysphagia by physical therapy. Depolarization of axons is induced by NMES, which induces swallowing muscle contraction and
improves swallowing (Bergquist etal., 2011). On the other hand, it can stimulate the excitability of the pharyngeal cortex, induce the operation of the
central pattern generator, and improve swallowing (Zeng etal., 2018). Sensory stimulation transmits signals through the cranial nerves, on the one hand,
directly to the central pattern generator, and on the other hand, it transmits signals to the cerebral cortex, where it is integrated and organized, and finally
promotes the swallowing response (Alvarez-Berdugo etal., 2016). A change in neural plasticity is used to accelerate the operation of swallowing circuits
through rTMS, which uses electromagnetic induction to depolarize synapses, ultimately improving swallowing (Labeit etal., 2024). Dysphagia can
beimproved by tDCS because it alters nerve cell polarity and triggers neuroplasticity (Speyer etal., 2022). NMES: Neuromuscular electrical stimulation;
tDCS, Transcranial direct current stimulation; rTMS, Repetitive transcranial magnetic stimulation, CPG, Central pattern generator; C, Carbonation.
Li et al. 10.3389/fnhum.2024.1404398
Frontiers in Human Neuroscience 05 frontiersin.org
powder (0.2 g/day) to the patient’s bilateral tongue using a cotton swab,
followed by swallowing practice instructions. Additionally, tongue
massage with the cotton swab and guidance for tongue and masticatory
muscle exercises were included (5–6 times per day, 15 min each time for
2 weeks). Acid stimulation promotes saliva secretion by stimulating the
tongue, thereby accelerating the swallowing process and relieving
swallowing disorders (Wang etal., 2022). Acid stimulation eectively
improves stroke dysphagia based on two fundamental principles.
Furthermore, acid stimulation increases the activity intensity of
swallowing-related muscles such as the mylohyoid and front belly of the
digastric muscles, triggering stronger contractions during swallowing
and consequently improving dysphagia (Palmer etal., 2005).
Carbonation stimulation is also considered to be a benecial
sensory stimulation technique for improving dysphagia, primarily by
enhancing the contractility of the velum and oropharynx (Omari etal.,
2020) as well as prolonging the opening duration of the upper
esophageal sphincter (Miura etal., 2009). A recent study conducted
by Morishita etal. (2023) has suggested that carbonation may induce
changes in brain excitability, resulting in shorter swallowing times in
healthy individuals consuming carbonated beverages. Additionally
Bülow et al. (2003) demonstrated that carbonation stimulation
eectively reduces airway aspiration and pharyngeal retention, and
shortens the duration of the pharyngeal phase duration. Sdravou etal.
(2012) conducted an experiment involving carbonation stimulation in
17 patients with neurogenic dysphagia, conrming that drinking
carbonated water can reduce aspiration. e eectiveness of
carbonation in improving swallowing function can beattributed to
two main factors. On the one hand, it is related to the activation of
swallowing pathways. Stimulation of peripheral sensory receptors and
sensory bers in the nucleus tractus solitarius in the brainstem
activates the pattern generator of the swallowing center (Nagano etal.,
2022). On the other hand, carbonation aects numerous receptors in
the larynx, namely mechanoreceptors, chemoreceptors, pain receptors,
and thermoreceptors, which respond to carbonation stimulation by
triggering protective reexes to prevent aspiration (Bradley, 2000).
In summary (see Figure 2), the intensity and duration of the
swallowing response can be triggered or regulated by a complex
biofeedback mechanism, sensory stimulation transmit signals mainly
through the trigeminal, facial, glossopharyngeal and vagus nerves,
which on the one hand directly reach the swallowing central pattern
generator, and on the other hand, transmit signals to the cerebral
cortex, which outputs information to the swallowing central pattern
generator for integration and tissue, the swallowing response is
facilitated (Alvarez-Berdugo etal., 2016).
3.3 Repetitive transcranial magnetic
stimulation
e technique of rTMS involves placing a coil to the head to
generate a magnetic eld when an electric current passes through it, this
magnetic eld induces current ows within brain tissue perpendicular
to its direction, which are of strong strength to induce modications in
both cortical and subcortical white matter axons (Ridding and Rothwell,
2007). e rTMS can either suppress or excite neuronal activity
depending on the frequency used: frequencies at or below 1 Hz suppress
neuronal activity, while those above 5 Hz elicit neuronal excitation
(Honda etal., 2021). Studies on healthy participants have investigated
the eects of rTMS on the pharyngeal motor cortex (Yamamura etal.,
2018). Findings indicate that rTMS at 1 Hz inhibits the excitability of
the pharyngeal motor cortex, whereas high-frequency stimulation, such
as 10 Hz stimulation of the cerebellar hemisphere increases the
amplitude of pharynx cortical motor evoked potentials (Vasant etal.,
2015; Sasegbon et al., 2020b). Due to its potential neural repair
mechanisms, dysphagia has been treated extensively with rTMS
(Sasegbon et al., 2020a). Recent schemes for rTMS treatment of
dysphagia are summarized in Table S2. According to Table S2, treatment
with rTMS focuses primarily on the cerebellum and pharyngeal motor
cortex. High frequencies are predominantly used for treatment
frequency, and the treatment time is mostly selected daily, 5 days a week,
for a total of 2 weeks, and the swallowing function test results are
improved (Dong etal., 2022; Rao etal., 2022; Zhong etal., 2023).
ere is evidence that rTMS alters cortical excitability, regulates
neurotransmitter release, and promotes neuroplasticity in the brain
(Kesikburun, 2022). e increase in cortical activity in the cerebral
hemispheres is associated with functional recovery in stroke patients
with dysphagia, and the reorganization of neural networks plays a
signicant role in the recovery of swallowing function (Hoogendam
etal., 2010). e changes in neuroplasticity are closely related to rTMS
induced synaptic connections in the process of regulating the
functional state of the cerebral cortex (Li etal., 2022). ere is no
single target for rTMS treatment of stroke dysphagia; rather, it involves
the regeneration of swallowing function in stroke patients through the
cooperative action of multiple brain areas (Dong etal., 2022). Aer
virtual lesion simulation in stroke patients with dysphagia, cerebellar
high frequency rTMS not only improves the excitability of the
pharyngeal motor cortex in healthy volunteers (Sasegbon etal., 2019,
2020b) but also improves swallowing function among stroke patients
with dysphagia (Zhong etal., 2023). is may beexplained by the fact
that the cerebellum is connected to the brainstem by three cerebellar
peduncles, which directly communicate with the various motor nuclei
of the brainstem (Roostaei etal., 2014). Based on the evidence that
rTMS could improve not only swallowing disorder but also motor
function, Khedr etal. applied rTMS to Parkinson’s disease patients
with dysphagia and achieved the envisaged results: Parkinson’s disease
patients could benet from rTMS for dysphagia (Khedr etal., 2019).
In summary (see Figure2), the cerebral cortex and cerebellum are
the primary stimulation targets for rTMS in dysphagia, it uses
electromagnetic induction to depolarization synapses and accelerate the
operation of swallowing circuit through changes in neuroplasticity, so as
to improve swallowing function (Speyer etal., 2022; Labeit etal., 2024).
3.4 Transcranial direct current stimulation
e tDCS technique is a groundbreaking method of non-invasive
brain stimulation (Pisegna etal., 2016) that involves applying small
electrical currents (1–2 mA) through two surface electrodes, the
anode electrode and cathode electrode, to targeted brain regions,
thereby triggering and modulating brain activity (He etal., 2022). In
recent years, there has been strong interest in tDCS as an eective,
noninvasive method to treat dysphagia (Cheng etal., 2021). Table S3
shows the application of tDCS in dysphagia treatment in recent years.
It can beseen from the table that tDCS is mostly used for stroke-
related dysphagia. e location of the anode is related to the area
involved in the pharyngeal motor cortex. e cathodes are mostly
Li et al. 10.3389/fnhum.2024.1404398
Frontiers in Human Neuroscience 06 frontiersin.org
placed in the contralateral supraorbital region and the opposite
shoulder, most single intervention sessions last 20 min, the treatment
eect can generally achieve the improvement of swallowing function
(Farpour etal., 2023; El Nahas etal., 2024).
Neuroplasticity is the concept behind tDCS, which is a form of
noninvasive brain stimulation (Kesikburun, 2022). e swallowing
motor task-related activities of the brain are enhanced through
glutamatergic and calcium-dependent processes, including
synaptogenesis, reorganization, strengthening, and inhibition of brain
networks (Pisegna etal., 2016; Tedesco Triccas etal., 2016). Anodal
tDCS can improve swallowing function by stimulating the pharyngeal
motor cortex in patients with Parkinson’s disease, which is associated
with an increase in the strength of synaptic connections related to
deglutition in the cerebral cortex (Tedesco Triccas et al., 2016).
Dysphagia can also beameliorated by tDCS in older adults with and
without neurological conditions, which is associated with the induction
of a polar-dependent shi in underlying cortical excitability, as well as
a broad activation of pharyngeal motor cortex in both brain hemispheres
(Cosentino etal., 2020). Furthermore, stroke patients oen choose
tDCS as a treatment for dysphagia, with numerous therapeutic targets
(Gómez-García et al., 2023), related to the regions involved in the
swallowing network and the fact that tDCS promotes neuroplasticity
(Ahn et al., 2017). For example, enhancing the excitability of the
uninjured side of the swallowing cortex, the injured side of the
swallowing cortex, the bilateral swallowing cortex, and the superior
limbic gyrus can be benecial for enhancing swallowing function
(Wang etal., 2020; Mao etal., 2022; Farpour etal., 2023). Dysphagia in
stroke patients can be eectively improved by tDCS, and anodal
stimulation of the right swallowing cortex in patients with multiple
sclerosis dysphagia can also improve swallowing function, as anodal
stimulation of the swallowing motor cortex of the right also activates an
extensive network involving the contralateral hemisphere to compensate
for the damage caused by focal brain injury (Cosentino etal., 2018).
In general (see Figure2), the treatment principle of tDCS can
improve dysphagia in a variety of diseases (Lefaucheur, 2016). Its
current directly stimulates the brain or cerebellum and changes the
polarity of nerve cells, aiming to trigger and promote neuroplasticity
and improve dysphagia (Speyer etal., 2022; Labeit etal., 2024).
4 Summary and prospect
Neurogenic dysphagia is currently managed with motor training,
oral medications, and surgery (El Halabi et al., 2023). Clinical
evidence supports the use of movement training for mild-to-
moderate dysphagia (Saconato et al., 2016), with strong
recommendations from intermediate and high-level evidence
sources (Medicine DRCO, 2023; Yang etal., 2023). Patients with
severe dysphagia typically undergo medical or surgical interventions,
especially at intermediate and advanced disease stages (Cotaoco
etal., 2024). While conventional treatments may take longer to reach
full ecacy, physical therapy has emerged as a non-invasive and
practical approach to shorten treatment duration for dysphagia
patients (Frost et al., 2018; Bengisu et al., 2024). is review
elucidates the physiological function of the swallowing system, the
pathological mechanisms of neurogenic dysphagia, and the
principles and application of physical therapy. Overall, physical
therapy oers benets to individuals with neurogenic dysphagia by
enhancing swallowing recovery, improving treatment outcomes, and
enhancing quality of life (Banda etal., 2023; Bengisu etal., 2024).
Considering the increasing public interest in neurogenic
dysphagia, it is crucial to also address its eects on individuals with
schizophrenia. Future research should prioritize investigating the
pathogenesis of dysphagia in schizophrenia, exploring the eectiveness
of physical therapy interventions, and identifying the most suitable
therapy targets for this specic population.
Author contributions
KL: Conceptualization, Writing – review & editing, Funding
acquisition, Supervision. CF: Writing – review & editing,
Conceptualization, Writing – original dra. ZX: Writing – review &
editing. JZ: Writing – original dra. CZ: Investigation, Writing –
original dra. RL: Investigation, Writing – original dra. CG:
Supervision, Writing – review & editing. JW: Writing – original dra.
CX: Writing – review & editing, Investigation. YZ: Writing – review
& editing. WD: Writing – review & editing.
Funding
e author(s) declare that nancial support was received for the
research, authorship, and/or publication of this article. is study was
supported by Medical and Health Science and Technology
Development Plan of Shandong Province (No. 202303090378); Key
Research Plan of Jining City (No. 2023YXNS006).
Acknowledgments
We appreciate the help of the Home for Researchers website in
drawing, writing, etc.
Conflict of interest
e authors declare that the research was conducted in the
absence of any commercial or nancial relationships that could
beconstrued as a potential conict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors
and do not necessarily represent those of their aliated organizations,
or those of the publisher, the editors and the reviewers. Any product
that may beevaluated in this article, or claim that may bemade by its
manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
e Supplementary material for this article can befound online
at: https://www.frontiersin.org/articles/10.3389/fnhum.2024.1404398/
full#supplementary-material
Li et al. 10.3389/fnhum.2024.1404398
Frontiers in Human Neuroscience 07 frontiersin.org
References
Ahn, Y., Sohn, H., Park, J., Ahn, T., Shin, Y., Park, M., et al. (2017). Eect of
bihemispheric anodal transcranial direct current stimulation for dysphagia in chronic
stroke patients: a randomized clinical trial. J. Rehabil. Med. 49, 30–35. doi:
10.2340/16501977-2170
Alfonsi, E., Bergamaschi, R., Cosentino, G., Ponzio, M., Montomoli, C., Restivo, D. A.,
et al. (2013). Electrophysiological patterns of oropharyngeal swallowing in multiple
sclerosis. Clin. Neurophysiol. 124, 1638–1645. doi: 10.1016/j.clinph.2013.03.003
Alvarez-Berdugo, D., Rofes, L., Casamitjana, J., Padrón, A., Quer, M., and Clavé, P.
(2016). Oropharyngeal and laryngeal sensory innervation in the pathophysiology of
swallowing disorders and sensory stimulation treatments. Ann. N. Y. Acad. Sci. 1380,
104–120. doi: 10.1111/nyas.13150
Ansari, N. N., Tarameshlu, M., and Ghelichi, L. (2020). Dysphagia in multiple sclerosis
patients: diagnostic and evaluation strategies. Degener. Neurol. Neuromuscul. Dis. 10,
15–28. doi: 10.2147/DNND.S198659
Banda, K. J., Wu, K.-C., Jen, H.-J., Chu, H., Pien, L.-C., Chen, R., et al. (2023).
Comparative eectiveness of combined and single Neurostimulation and traditional
dysphagia therapies for post-stroke dysphagia: a network Meta-analysis. Neurorehabil.
Neural Repair 37, 194–204. doi: 10.1177/15459683231166940
Bath, P. M., Lee, H. S., and Everton, L. F. (2018). Swallowing therapy for dysphagia in
acute and subacute stroke. Cochrane Database Syst. Rev. 2018:CD000323. doi:
10.1002/14651858.CD000323.pub3
Bengisu, S., Demir, N., and Krespi, Y. (2024). Eectiveness of conventional dysphagia
therapy (CDT), neuromuscular electrical stimulation (NMES), and transcranial direct
current stimulation (tDCS) in acute post-stroke dysphagia: a comparative evaluation.
Dysphagia 39, 77–91. doi: 10.1007/s00455-023-10595-w
Beom, J., Kim, S. J., and Han, T. R. (2011). Electrical stimulation of the suprahyoid
muscles in brain-injured patients with dysphagia: a pilot study. Ann. Rehabil. Med. 35,
322–327. doi: 10.5535/arm.2011.35.3.322
Bergquist, A. J., Clair, J. M., Lagerquist, O., Mang, C. S., Okuma, Y., and Collins, D. F.
(2011). Neuromuscular electrical stimulation: implications of the electrically evoked
sensory volley. Eur. J. Appl. Physiol. 111, 2409–2426. doi: 10.1007/s00421-011-2087-9
Bradley, R. M. (2000). Sensory receptors of the larynx. Am. J. Med. 108, 47–50. doi:
10.1016/s0002-9343(99)00339-3
Bülow, M., Olsson, R., and Ekberg, O. (2003). Videoradiographic analysis of how
carbonated thin liquids and thickened liquids aect the physiology of swallowing in
subjects with aspiration on thin liquids. Acta Radiol. 44, 366–372. doi:
10.1080/j.1600-0455.2003.00100.x
Carnaby, G., LaGorio, L., Silliman, S., and Crary, M. (2020). Exercise-based swallowing
intervention (McNeill dysphagia therapy) with adjunctive NMES to treat dysphagia post-stroke:
a double-blind placebo-controlled trial. J. Oral Rehabil. 47, 501–510. doi: 10.1111/joor.12928
Carson, R. G., and Buick, A. R. (2021). Neuromuscular electrical stimulation-promoted
plasticity of the human brain. J. Physiol. 599, 2375–2399. doi: 10.1113/JP278298
Chandran, S. K., and Doucet, M. (2024). Neurogenic dysphagia. Otolaryngol. Clin. N.
Am. 2024:23. doi: 10.1016/j.otc.2024.02.023
Chen, Q., Kan, M., Jiang, X., Liu, H., Zhang, D., Yuan, L., et al. (2024). Comparison of
the ecacy and tolerability of dierent repetitive transcranial magnetic stimulation
modalities for post-stroke dysphagia: a systematic review and Bayesian network meta-
analysis protocol. BMJ Open 14:e080289. doi: 10.1136/bmjopen-2023-080289
Cheng, I., Sasegbon, A., and Hamdy, S. (2021). Eects of Neurostimulation on
Poststroke dysphagia: a synthesis of current evidence from randomized controlled trials.
Neuromodulation 24, 1388–1401. doi: 10.1111/ner.13327
Cheng, I., Takahashi, K., Miller, A., and Hamdy, S. (2022). Cerebral control of
swallowing: an update on neurobehavioral evidence. J. Neurol. Sci. 442:120434. doi:
10.1016/j.jns.2022.120434
Cola, P., Gatto, A., da Silva, R., Spadotto, A., Ribeiro, P., Schelp, A., et al. (2012). Taste
and temperature in swallowing transit time aer stroke. Cerebrovasc. Dis. Extra 2, 45–51.
doi: 10.1159/000339888
Cosentino, G., Gargano, R., Bonura, G., Realmuto, S., Tocco, E., Ragonese, P., et al. (2018).
Anodal tDCS of the swallowing motor cortex for treatment of dysphagia in multiple sclerosis:
a pilot open-label study. Neurol. Sci. 39, 1471–1473. doi: 10.1007/s10072-018-3443-x
Cosentino, G., Tassorelli, C., Prunetti, P., Bertino, G., De Icco, R., Todisco, M., et al.
(2020). Anodal transcranial direct current stimulation and intermittent theta-burst
stimulation improve deglutition and swallowing reproducibility in elderly patients with
dysphagia. Neurogastroenterol. Motil. 32:e13791. doi: 10.1111/nmo.13791
Costa, M. M. B. (2018). NEURAL CONTROL OF SWALLOWING. Arq. Gastroenterol.
55, 61–75. doi: 10.1590/S0004-2803.201800000-45
Cotaoco, C., Ueha, R., Koyama, M., Sato, T., Goto, T., and Kondo, K. (2024).
Swallowing improvement surgeries. Eur. Arch. Otorhinolaryngol. 281, 2807–2817. doi:
10.1007/s00405-024-08452-z
Cui, F., Yin, Q., Wu, C., Shen, M., Zhang, Y., Ma, C., et al. (2020). Capsaicin combined
with ice stimulation improves swallowing function in patients with dysphagia aer
stroke: a randomised controlled trial. J. Oral Rehabil. 47, 1297–1303. doi: 10.1111/
joor.13068
Dashtelei, A. A., Nitsche, M. A., Salehinejad, M. A., Habibi, A. H., Bakhtyiari, J., and
Khatoonabadi, A. R. (2024). Adjunctive transcranial direct current stimulation to
improve swallowing functions in Parkinson's disease. EXCLI J. 23, 95–107. doi:
10.17179/excli2023-6496
Dodds, W., Stewart, E., and Logemann, J. (1990). Physiology and radiology of the
normal oral and pharyngeal phases of swallowing. AJR Am. J. Roentgenol. 154, 953–963.
doi: 10.2214/ajr.154.5.2108569
Dong, L., Pan, X., Wang, Y., Bai, G., Han, C., Wang, Q., et al. (2022). High-frequency
cerebellar rTMS improves the swallowing function of patients with dysphagia aer
brainstem stroke. Neural Plast. 2022, 6259693–6259699. doi: 10.1155/2022/6259693
Doucet, B., Lam, A., and Grin, L. (2012). Neuromuscular electrical stimulation for
skeletal muscle function. Yale J. Biol. 85, 201–215.
El Halabi, M., Arwani, R., and Parkman, H. (2023). Dysphagia in neurological
disorders. Semin. Neurol. 43, 530–539. doi: 10.1055/s-0043-1771458
El Nahas, N., Shokri, H., Refaat, A., Mousa, H., Hamid, A., Abdel Monem, A., et al.
(2024). e eect of transcranial direct current stimulation paired with neuromuscular
electrical stimulation on swallowing function in post stroke dysphagia. Egypt. J. Neurol.
Psychiatry Neurosurg. 60:21. doi: 10.1186/s41983-023-00767-8
Farpour, S., Asadi-Shekaari, M., Borhani Haghighi, A., and Farpour, H. (2023).
Improving swallowing function and ability in post stroke dysphagia: a randomized
clinical trial. Dysphagia 38, 330–339. doi: 10.1007/s00455-022-10470-0
Ferrara, L., Kamity, R., Islam, S., Sher, I., Barlev, D., Wennerholm, L., et al. (2018).
Short-term eects of cold liquids on the pharyngeal swallow in preterm infants with
dysphagia: a pilot study. Dysphagia 33, 593–601. doi: 10.1007/s00455-018-9877-8
Frost, J., Robinson, H. F., and Hibberd, J. (2018). A comparison of neuromuscular
electrical stimulation and traditional therapy, versus traditional therapy in patients with
longstanding dysphagia. Curr. Opin. Otolaryngol. Head Neck Surg. 26, 167–173. doi:
10.1097/MOO.0000000000000454
Gómez-García, N., Álvarez-Barrio, L., Leirós-Rodríguez, R., Soto-Rodríguez, A.,
Andrade-Gómez, E., and Hernández-Lucas, P. (2023). Transcranial direct current
stimulation for post-stroke dysphagia: a meta-analysis. J. Neuroeng. Rehabil. 20:165. doi:
10.1186/s12984-023-01290-w
Hashimoto, K., Sugiyama, Y., Fuse, S., Umezaki, T., Oku, Y., Dutschmann, M., et al.
(2019). Activity of swallowing-related neurons in the medulla in the perfused brainstem
preparation in rats. Laryngoscope 129, E72–E79. doi: 10.1002/lary.27401
He, K., Wu, L., Huang, Y., Chen, Q., Qiu, B., Liang, K., et al. (2022). Ecacy and safety
of transcranial direct current stimulation on post-stroke dysphagia: a systematic review
and Meta-analysis. J. Clin. Med. 11:297. doi: 10.3390/jcm11092297
Honda, Y., Nakamura, S., Ogawa, K., Yoshino, R., Tobler, P., Nishimura, Y., et al.
(2021). Changes in beta and high-gamma power in resting-state electrocorticogram
induced by repetitive transcranial magnetic stimulation of primary motor cortex in
unanesthetized macaque monkeys. Neurosci. Res. 171, 41–48. doi: 10.1016/j.
neures.2021.02.002
Hoogendam, J. M., Ramakers, G. M. J., and Di Lazzaro, V. (2010). Physiology of
repetitive transcranial magnetic stimulation of the human brain. Brain Stimul. 3, 95–118.
doi: 10.1016/j.brs.2009.10.005
Hurtte, E., Young, J., and Gyawali, C. (2023). Dysphagia. Prim. Care 50, 325–338. doi:
10.1016/j.pop.2023.03.001
Ilott, I., Gerrish, K., Eltringham, S. A., Taylor, C., and Pownall, S. (2016).
Exploring factors that influence the spread and sustainability of a dysphagia
innovation: an instrumental case study. BMC Health Serv. Res. 16:406. doi: 10.1186/
s12913-016-1653-6
Jang, S. H., and Kim, M. S. (2021). Dysphagia in lateral medullary syndrome: a
narrative review. Dysphagia 36, 329–338. doi: 10.1007/s00455-020-10158-3
Jean, A. (2001). Brain stem control of swallowing: neuronal network and cellular
mechanisms. Physiol. Rev. 81, 929–969. doi: 10.1152/physrev.2001.81.2.929
Jeon, Y. H., Cho, K. H., and Park, S. J. (2020). Eects of neuromuscular electrical
stimulation (NMES) plus upper cervical spine mobilization on forward head posture
and swallowing function in stroke patients with dysphagia. Brain Sci. 10:478. doi:
10.3390/brainsci10080478
Kawakami, M., Simeoni, S., Tremblay, S., Hannah, R., Fujiwara, T., and Rothwell, J.
(2019). Changes in the excitability of corticobulbar projections due to intraoral cooling
with ice. Dysphagia 34, 708–712. doi: 10.1007/s00455-018-09975-4
Kesikburun, S. (2022). Non-invasive brain stimulation in rehabilitation. Turk. J. Phys.
Med. Rehabil. 68, 1–8. doi: 10.5606/trd.2022.10608
Khedr, E. M., Mohamed, K. O., Soliman, R. K., Hassan, A. M. M., and
Rothwell, J. C. (2019). e eect of high-frequency repetitive transcranial magnetic
stimulation on advancing Parkinson's disease with dysphagia: double blind
randomized clinical trial. Neurorehabil. Neural Repair 33, 442–452. doi:
10.1177/1545968319847968
Kocica, J., Lasotova, N., Kolcava, J., Svobodova, M., Hladikova, M., Stourac, P., et al.
(2024). Screening for dysphagia in patients with relapsing-remitting multiple sclerosis.
Mult. Scler. Relat. Disord. 83:105418. doi: 10.1016/j.msard.2023.105418
Li et al. 10.3389/fnhum.2024.1404398
Frontiers in Human Neuroscience 08 frontiersin.org
Konecny, P., and Elfmark, M. (2018). Electrical stimulation of hyoid muscles in post-
stroke dysphagia. Biomed. Pap. Med. Fac. Univ. Palacky Olomouc Czec. Repub. 162,
40–42. doi: 10.5507/bp.2017.043
Labeit, B., Claus, I., Muhle, P., Lapa, S., Suntrup-Krueger, S., Dziewas, R., et al. (2020a).
Oropharyngeal freezing and its relation to dysphagia- an analogy to freezing of gait.
Parkinsonism Relat. Disord. 75, 1–6. doi: 10.1016/j.parkreldis.2020.05.002
Labeit, B., Claus, I., Muhle, P., Suntrup-Krueger, S., Dziewas, R., and Warnecke, T.
(2020b, 2020). Eect of intestinal levodopa-carbidopa infusion on pharyngeal dysphagia:
results from a retrospective pilot study in patients with Parkinson's disease. Parkinsons
Dis. 2020:4260501. doi: 10.1155/2020/4260501
Labeit, B., Jung, A., Ahring, S., Oelenberg, S., Muhle, P., Roderigo, M., et al. (2023).
Relationship between post-stroke dysphagia and pharyngeal sensory impairment.
Neurol. Res. Pract. 5:7. doi: 10.1186/s42466-023-00233-z
Labeit, B., Michou, E., Trapl-Grundschober, M., Suntrup-Krueger, S., Muhle, P.,
Bath, P. M., et al. (2024). Dysphagia aer stroke: research advances in treatment
interventions. Lancet Neurol. 23, 418–428. doi: 10.1016/S1474-4422(24)00053-X
Lefaucheur, J.-P. (2016). A comprehensive database of published tDCS clinical trials
(2005-2016). Clin. Neurophysiol. 46, 319–398. doi: 10.1016/j.neucli.2016.10.002
Li, Y., Chen, K., Wang, J., Lu, H., Li, X., Yang, L., et al. (2022). Research progress on
transcranial magnetic stimulation for post-stroke dysphagia. Front. Behav. Neurosci.
16:995614. doi: 10.3389/fnbeh.2022.995614
Li, W., Kang, X., Ren, J., Lai, X., and Tai, L. (2017). Eects of extended in-patient
treatment training on outcome of post-stroke dysphagia. Eur. Rev. Med. Pharmacol. Sci.
21, 5711–5716. doi: 10.26355/eurrev_201712_14017
Li, K.-P., Wu, J.-J., Zhou, Z.-L., Xu, D.-S., Zheng, M.-X., Hua, X.-Y., et al. (2023).
Noninvasive brain stimulation for neurorehabilitation in post-stroke patients. Brain Sci.
13:30451. doi: 10.3390/brainsci13030451
Logemann, J., Pauloski, B., Colangelo, L., Lazarus, C., Fujiu, M., and Kahrilas, P.
(1995). Eects of a sour bolus on oropharyngeal swallowing measures in patients with
neurogenic dysphagia. J. Speec Hear. Res. 38, 556–563. doi: 10.1044/jshr.3803.556
Mao, H., Lyu, Y., Li, Y., Gan, L., Ni, J., Liu, L., et al. (2022). Clinical study on swallowing
function of brainstem stroke by tDCS. Neurol. Sci. 43, 477–484. doi: 10.1007/
s10072-021-05247-6
McCarty, E. B., and Chao, T. N. (2021). Dysphagia and swallowing disorders. Med.
Clin. North Am. 105, 939–954. doi: 10.1016/j.mcna.2021.05.013
Medicine DRCO (2023). Chinese guidelines for the rehabilitation management of
dysphagia (2023 edition). Chin. J. Phys. Med. Rehabil. 45, 1057–1072. doi: 10.3760/cma
.j.issn.0254-1424.2023.12.001
Meng, P., Zhang, S., Wang, Q., Wang, P., Han, C., Gao, J., et al. (2018). e eect of
surface neuromuscular electrical stimulation on patients with post-stroke dysphagia. J.
Back Musculoskelet. Rehabil. 31, 363–370. doi: 10.3233/bmr-170788
Miller, S., Peters, K., and Ptok, M. (2022). Review of the eectiveness of neuromuscular
electrical stimulation in the treatment of dysphagia- an update. Ger. Med. Sci. 20:Doc08.
doi: 10.3205/000310
Miura, Y., Morita, Y., Koizumi, H., and Shingai, T. (2009). Eects of taste solutions,
carbonation, and cold stimulus on the power frequency content of swallowing submental
surface electromyography. Chem. Senses 34, 325–331. doi: 10.1093/chemse/bjp005
Morishita, M., Sota, J., and Kobayashi, M. (2023). Eects of carbonated beverages on
sustained swallowing behavior changes in older inpatients. Physiol. Behav. 265:114172.
doi: 10.1016/j.physbeh.2023.114172
Nagano, A., Maeda, K., Shimizu, A., Murotani, K., and Mori, N. (2022). Eects of
carbonation on swallowing: systematic review and Meta-analysis. Laryngoscope 132,
1924–1933. doi: 10.1002/lary.30019
Nakamura, T., and Fujishima, I. (2013). Usefulness of ice massage in triggering the
swallow reex. J. Stroke Cerebrovasc. Dis. 22, 378–382. doi: 10.1016/j.
jstrokecerebrovasdis.2011.09.016
Nascimento, W., Tomsen, N., Acedo, S., Campos-Alcantara, C., Cabib, C.,
Alvarez-Larruy, M., et al. (2021). Eect of aging, gender and sensory stimulation of
TRPV1 receptors with capsaicin on spontaneous swallowing frequency in patients with
oropharyngeal dysphagia: a proof-of-concept study. Diagnostics (Basel, Switzerland)
11:461. doi: 10.3390/diagnostics11030461
Oh, J.-C. (2016). Eects of tongue-hold swallows on suprahyoid muscle activation
according to the relative tongue protrusion length: a preliminary study. Springerplus
5:1144. doi: 10.1186/s40064-016-2799-8
Oh, D., Park, J., Kim, H., Chang, M., and Hwang, N. (2020). e eect of
neuromuscular electrical stimulation with dierent electrode positions on swallowing
in stroke patients with oropharyngeal dysphagia: a randomized trial. J. Back
Musculoskelet. Rehabil. 33, 637–644. doi: 10.3233/bmr-181133
Omari, T., Ciucci, M., Gozdzikowska, K., Hernández, E., Hutcheson, K., Jones, C.,
et al. (2020). High-resolution pharyngeal manometry and impedance: protocols and
metrics-recommendations of a high-resolution pharyngeal manometry international
working group. Dysphagia 35, 281–295. doi: 10.1007/s00455-019-10023-y
Palmer, P., McCulloch, T., Jae, D., and Neel, A. (2005). Eects of a sour bolus on the
intramuscular electromyographic (EMG) activity of muscles in the submental region.
Dysphagia 20, 210–217. doi: 10.1007/s00455-005-0017-x
Panebianco, M., Marchese-Ragona, R., Masiero, S., and Restivo, D. A. (2020).
Dysphagia in neurological diseases: a literature review. Neurol. Sci. 41, 3067–3073. doi:
10.1007/s10072-020-04495-2
Park, J., Oh, D., Hwang, N., and Lee, J. (2018). Eects of neuromuscular electrical
stimulation in patients with Parkinson's disease and dysphagia: a randomized, single-
blind, placebo-controlled trial. NeuroRehabilitation 42, 457–463. doi: 10.3233/nre-172306
Patel, B., Legacy, J., Hegland, K. W., Okun, M. S., and Herndon, N. E. (2020). A comprehensive
review of the diagnosis and treatment of Parkinson's disease dysphagia and aspiration. Expert
Rev. Gastroenterol. Hepatol. 14, 411–424. doi: 10.1080/17474124.2020.1769475
Pelletier, C., and Lawless, H. (2003). Eect of citric acid and citric acid-sucrose
mixtures on swallowing in neurogenic oropharyngeal dysphagia. Dysphagia 18,
231–241. doi: 10.1007/s00455-003-0013-y
Petko, B., and Tadi, P. (2023). Neuroanatomy, nucleus Ambiguus. Hoboken, NJ: Wiley.
Pisegna, J., Kaneoka, A., Pearson, W., Kumar, S., and Langmore, S. (2016). Eects of
non-invasive brain stimulation on post-stroke dysphagia: a systematic review and meta-
analysis of randomized controlled trials. Clin. Neurophysiol. 127, 956–968. doi: 10.1016/j.
clinph.2015.04.069
Qin, Y., Tang, Y., Liu, X., and Qiu, S. (2023). Neural basis of dysphagia in stroke: a
systematic review and meta-analysis. Front. Hum. Neurosci. 17:1077234. doi: 10.3389/
fnhum.2023.1077234
Qin, L., Zhang, X.-P., Yang, X.-C., Cui, C.-H., Shi, J., and Jia, C.-S. (2019). Deep
acupuncture of Lianquan (CV23) and Yifeng (TE17) in combination with conventional
acupuncture of other acupoints is superior to swallowing rehabilitation training in
improving post-stroke dysphagia in apoplexy patients. Zhen Ci Yan Jiu 44, 144–147. doi:
10.13702/j.1000-0607.180018
Rao, J., Li, F., Zhong, L., Wang, J., Peng, Y., Liu, H., et al. (2022). Bilateral cerebellar
intermittent eta burst stimulation combined with swallowing speech therapy for
dysphagia aer stroke: a randomized, double-blind, sham-controlled, Clinical Trial.
Neurorehabil. Neural Repair 36, 437–448. doi: 10.1177/15459683221092995
Regan, J. (2020). Impact of sensory stimulation on Pharyngo-esophageal swallowing
biomechanics in adults with dysphagia: a high-resolution manometry study. Dysphagia
35, 825–833. doi: 10.1007/s00455-019-10088-9
Ridding, M., and Rothwell, J. (2007). Is there a future for therapeutic use of
transcranial magnetic stimulation? Nat. Rev. Neurosci. 8, 559–567. doi: 10.1038/nrn2169
Rofes, L., Arreola, V., Almirall, J., Cabré, M., Campins, L., García-Peris, P., et al. (2011).
Diagnosis and management of oropharyngeal dysphagia and its nutritional and respiratory
complications in the elderly. Gastroenterol. Res. Pract. 2011, 1–13. doi: 10.1155/2011/818979
Roostaei, T., Nazeri, A., Sahraian, M. A., and Minagar, A. (2014). e human
cerebellum: a review of physiologic neuroanatomy. Neurol. Clin. 32, 859–869. doi:
10.1016/j.ncl.2014.07.013
Saconato, M., Chiari, B. M., Lederman, H. M., and Gonçalves, M. I. R. (2016).
Eectiveness of Chin-tuck maneuver to facilitate swallowing in neurologic dysphagia.
Int. Arch. Otorhinolaryngol. 20, 13–17. doi: 10.1055/s-0035-1564721
Sasegbon, A., Cheng, I., and Hamdy, S. (2024). e neurorehabilitation of post-stroke
dysphagia: physiology and pathophysiology. J. Physiol. doi: 10.1113/JP285564
Sasegbon, A., Cheng, I., Zhang, M., and Hamdy, S. (2020a). Advances in the use of
neuromodulation for neurogenic dysphagia: mechanisms and therapeutic application
of pharyngeal electrical stimulation, transcranial magnetic stimulation, and transcranial
direct current stimulation. Am. J. Speech Lang. Pathol. 29, 1044–1064. doi: 10.1044/2020_
AJSLP-19-00073
Sasegbon, A., and Hamdy, S. (2023). e role of the cerebellum in swallowing.
Dysphagia 38, 497–509. doi: 10.1007/s00455-021-10271-x
Sasegbon, A., Smith, C., Bath, P., Rothwell, J., and Hamdy, S. (2020b). e eects of
unilateral and bilateral cerebellar rTMS on human pharyngeal motor cortical activity and
swallowing behavior. Exp. Brain Res. 238, 1719–1733. doi: 10.1007/s00221-020-05787-x
Sasegbon, A., Watanabe, M., Simons, A., Michou, E., Vasant, D., Magara, J., et al.
(2019). Cerebellar repetitive transcranial magnetic stimulation restores pharyngeal brain
activity and swallowing behaviour aer disruption by a cortical virtual lesion. J. Physiol.
597, 2533–2546. doi: 10.1113/jp277545
Sdravou, K., Walshe, M., and Dagdilelis, L. (2012). Eects of carbonated liquids on
oropharyngeal swallowing measures in people with neurogenic dysphagia. Dysphagia
27, 240–250. doi: 10.1007/s00455-011-9359-8
Seo, K.-H., Jang, J., Jang, E. G., Park, Y., Lee, S. Y., Kim, B. R., et al. (2021). Clinical
eectiveness of the sequential 4-channel NMES compared with that of the conventional
2-channel NMES for the treatment of dysphagia in a prospective double-blind randomized
controlled study. J. Neuroeng. Rehabil. 18:90. doi: 10.1186/s12984-021-00884-6
Shaw, S. M., and Martino, R. (2013). e normal swallow: muscular and neurophysiological
control. Otolaryngol. Clin. N. Am. 46, 937–956. doi: 10.1016/j.otc.2013.09.006
Speyer, R., Sutt, A.-L., Bergström, L., Hamdy, S., Pommée, T., Balaguer, M., et al.
(2022). Neurostimulation in people with oropharyngeal dysphagia: a systematic review
and Meta-analysis of randomised controlled trials-part II: brain Neurostimulation. J.
Clin. Med. 11:993. doi: 10.3390/jcm11040993
Tan, C., Liu, Y., Li, W., Liu, J., and Chen, L. (2013). Transcutaneous neuromuscular electrical
stimulation can improve swallowing function in patients with dysphagia caused by non-stroke
diseases: a meta-analysis. J. Oral Rehabil. 40, 472–480. doi: 10.1111/joor.12057
Li et al. 10.3389/fnhum.2024.1404398
Frontiers in Human Neuroscience 09 frontiersin.org
Tedesco Triccas, L., Burridge, J. H., Hughes, A. M., Pickering, R. M., Desikan, M.,
Rothwell, J. C., et al. (2016). Multiple sessions of transcranial direct current stimulation
and upper extremity rehabilitation in stroke: a review and meta-analysis. Clin.
Neurophysiol. 127, 946–955. doi: 10.1016/j.clinph.2015.04.067
Teismann, I., Steinstraeter, O., Stoeckigt, K., Suntrup, S., Wollbrink, A., Pantev, C.,
et al. (2007). Functional oropharyngeal sensory disruption interferes with the cortical
control of swallowing. BMC Neurosci. 8:62. doi: 10.1186/1471-2202-8-62
Vasant, D., Michou, E., Mistry, S., Rothwell, J., and Hamdy, S. (2015). High-frequency
focal repetitive cerebellar stimulation induces prolonged increases in human pharyngeal
motor cortex excitability. J. Physiol. 593, 4963–4977. doi: 10.1113/jp270817
Wang, J., Chang, E., and Jiang, Y. (2022). Eects of vitamin C stimulation on
rehabilitation of dysphagia aer stroke: a randomized trial. Eur. J. Phys. Rehabilitation
Med. 58, 558–564. doi: 10.23736/s1973-9087.22.07337-3
Wang, Z., Chen, J., Lin, Z., and Ni, G. (2020). Transcranial direct current stimulation
improves the swallowing function in patients with cricopharyngeal muscle dysfunction
following a brainstem stroke. Neurol. Sci. 41, 569–574. doi: 10.1007/s10072-019-04120-x
Wang, Z., Xiao, Z., Shen, Q., Zhao, N., and Zhang, W. (2023). Neuromuscular electrical
stimulation for post-stroke dysphagia treatment: a systemic evaluation and Meta-analysis of
randomized controlled trials. Dysphagia 39, 424–432. doi: 10.1007/s00455-023-10626-6
Warnecke, T., Labeit, B., Schroeder, J., Reckels, A., Ahring, S., Lapa, S., et al. (2021).
Neurogenic dysphagia: systematic review and proposal of a classication system.
Neurology 96, e876–e889. doi: 10.1212/WNL.0000000000011350
Xia, X., Zhang, W., Guo, J., Chang, X., Zhao, R., Wang, J., et al. (2023). Diagnostic utility
of dierent dysphagia screening tools to detect dysphagia in individuals with amyotrophic
lateral sclerosis. Neurol. Sci. 44, 3919–3927. doi: 10.1007/s10072-023-06918-2
Yamamoto, R., Sugiyama, Y., Hashimoto, K., Kinoshita, S., Takemura, A., Fuse, S., et al.
(2022). Firing characteristics of swallowing interneurons in the dorsal medulla during
physiologically induced swallowing in perfused brainstem preparation in rats. Neurosci.
Res. 177, 64–77. doi: 10.1016/j.neures.2021.11.006
Yamamura, K., Kurose, M., and Okamoto, K. (2018). Guide to enhancing swallowing
initiation: insights from ndings in healthy subjects and Dysphagic patients. Curr. Phys.
Med. Rehabil. Rep. 6, 178–185. doi: 10.1007/s40141-018-0192-y
Yang, S., Park, J.-W., Min, K., Lee, Y. S., Song, Y.-J., Choi, S. H., et al. (2023). Clinical
practice guidelines for oropharyngeal dysphagia. Ann. Rehabil. Med. 47, S1–S26. doi:
10.5535/arm.23069
Ye, Q., Yuan, S., Yao, L., Dai, Y., Deng, B., Hu, J., et al. (2024). Participation of the
nucleus tractus solitarius in the therapeutic eect of electroacupuncture on post-stroke
dysphagia through the primary motor cortex. CNS Neurosci. er. 30:e14442. doi:
10.1111/cns.14442
Zeng, Y., Yip, J., Cui, H., Guan, L., Zhu, H., Zhang, W., et al. (2018). Ecacy of
neuromuscular electrical stimulation in improving the negative psychological state in
patients with cerebral infarction and dysphagia. Neurol. Res. 40, 473–479. doi:
10.1080/01616412.2018.1451015
Zhang, Y., Dou, Z., Zhao, F., Xie, C., Shi, J., Yang, C., et al. (2022). Neuromuscular
electrical stimulation improves swallowing initiation in patients with post-stroke
dysphagia. Front. Neurosci. 16:1011824. doi: 10.3389/fnins.2022.1011824
Zhong, L., Wen, X., Liu, Z., Li, F., Ma, X., Liu, H., et al. (2023). Eects of bilateral
cerebellar repetitive transcranial magnetic stimulation in poststroke dysphagia: a
randomized sham-controlled trial. NeuroRehabilitation 52, 227–234. doi: 10.3233/
NRE-220268
